Abstract
Background:
Type 1 calreticulin (CALR) variants comprise ~70% of all CALR mutations in primary myelofibrosis (PMF) and form a distinct phenotypic and prognostic disease subset (Leukemia. 2014;28:1568). Determinants of long-term outcome have not, however, been systematically appraised in this population. The current study documents the natural history, molecular correlates, and independent predictors of overall (OS), leukemia-free (LFS), and thrombosis-free (TFS) survival in CALR type 1/like-mutated myelofibrosis.
Methods:
Patients were recruited from the Mayo Clinic, Rochester, MN, USA. Diagnoses were consistent with World Health Organization (PMF, fibrotic/leukemic transformations) (Blood. 2016;127:2391) and International Working Group for Myeloproliferative Neoplasms Research and Treatment criteria (post-essential thrombocythemia (ET) MF) (Leukemia. 2008;22:437). Laboratory and clinical data were retrospectively abstracted corresponding to time of referral (PMF) or myelofibrotic transformation (post-ET MF). Conventional prognostic scoring was as previously outlined (Blood. 2010;115:1703; J Clin Oncol. 2018;36:1769). Recipients of allogeneic stem cell transplant were censored at the time of transplant. Standard statistical methods were used for all analyses using the JMP® Pro 13.0.0 software package (SAS Institute, Cary, NC, USA).
Results:
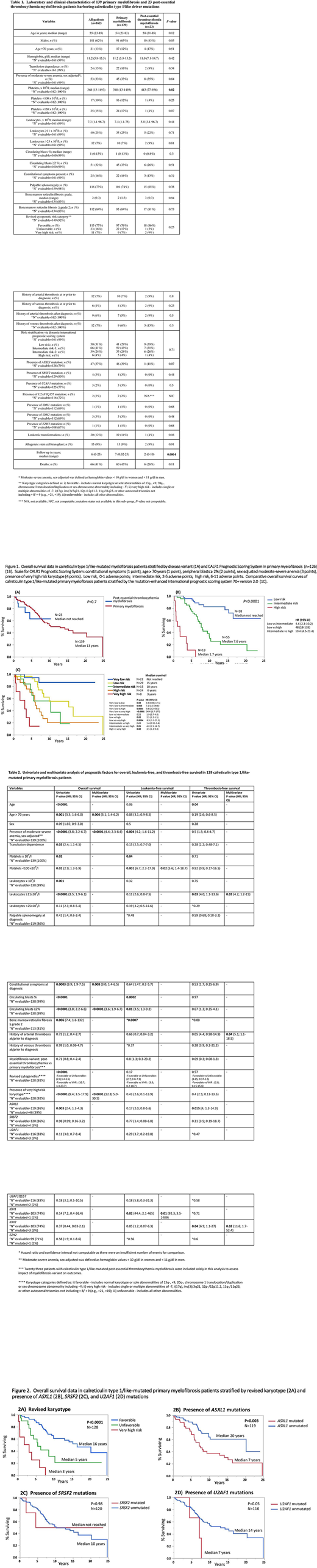
A total of 162 consecutive patients with CALR type 1/like-mutated myelofibrosis were identified: 139 (86%) with PMF and 23 (14%) with post-ET MF with median age 55 years (range 23-85), 62% male. The PMF and post-ET MF cohorts displayed similar phenotypic features with the exception of higher platelet counts (median 443 vs 340 x 109/l; P=0.02) in post-ET MF (Table 1). The most frequent co-existing mutations were ASXL1 (n=47; 37%) and SRSF2 (n=4; 3%), with ASXL1 seen more frequently in PMF (39% vs 11% post-ET MF; P=0.07). Over a median follow-up of 6 years (range 0-25 years), a total of 20 (12%) leukemic transformations and 66 (41%) deaths were recorded, with no significant differences between the PMF and post-ET MF cohorts (P=0.16 and 0.11, respectively). Kaplan-Meier survival estimates revealed comparably favorable median OS in both CALR type 1/like-mutated variants: not yet reached and 13 years in post-ET MF vs PMF, respectively (P=0.7) (Figure 1A).
Multivariable analysis disclosed moderate to severe sex-adjusted anemia (P<0.001), ≥2% circulating blasts (P<0.001), very high risk (VHR) karyotype (P<0.001), age >70 years (P=0.006), and constitutional symptoms (P=0.008) to be independent predictors of inferior OS in CALR type 1/like-mutated PMF (Table 2). LFS was significantly shortened in the presence of IDH1 mutations (P=0.01) and platelets <100 x 109/l (P=0.02) while IDH2 mutations (P=0.02), leukocytosis ≥11 x 109/l (P=0.03) and history of arterial thrombosis (P=0.04) were independent predictors of shortened TFS. Importantly, myelofibrosis variant (primary vs post-ET) did not influence survival (P=0.7) or complication rates (P=0.8 for LFS, P=0.09 for TFS) (Table 2).
The karyotype- and mutation-enhanced international prognostic scoring system (MIPSS70+ version 2.0), was effective in risk stratifying type 1/like CALR-mutated PMF (P-values 0.01 to <0.0001), with the exception of low vs intermediate (P=0.22) and intermediate vs high risk (P=0.49) (Figure 1C). The detrimental influences of unfavorable/VHR karyotype and ASXL1 mutations were confirmed (Figure 2A-B) while borderline adverse and prognostically neutral effects were seen on OS for U2AF1 (n=116; P=0.05) and SRSF2 (n=120; P=0.98) respectively (Figure 2C-D).
Conclusions:
The current study documents analogous disease patterns in primary and post-ET CALR type 1/like-mutated MF and provides information on determinants of long-term survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal